Abstract
Background: ITP has a multifaceted impact on patients' (pts) lives but there are limited data on which signs and symptoms treating physicians (MDs) and pts perceive as having the greatest impact, especially on pt quality of life (QoL). As in many chronic autoimmune diseases, fatigue can significantly affect QoL in ITP pts.
Aims: I-WISh studied the burden of ITP and its impact on QoL, especially fatigue, using a global pt and MD sampling frame. This analysis reports pt vs MD perception of frequency and severity of signs and symptoms.
Methods: I-WISh is a cross-sectional survey of ITP pts, recruited via MDs and pt support groups, and also of MDs, recruited via local fieldwork agencies. Participants (pts and MDs) completed a 30-minute online survey that included demographics, signs and symptoms, impact of symptoms, and pt-MD relationships. A steering committee of expert MDs and pt advocacy ITP specialists designed and endorsed survey materials.
Results: 1491 pts from 12 countries completed the survey; 65% female, with a mean (SD) age of 47 (16) yrs. 472 MDs from 13 countries completed the survey, with a mean (SD) ITP pt caseload of 34 (50) and a mean (SD) of 18 (36) newly-diagnosed pts in the past yr.
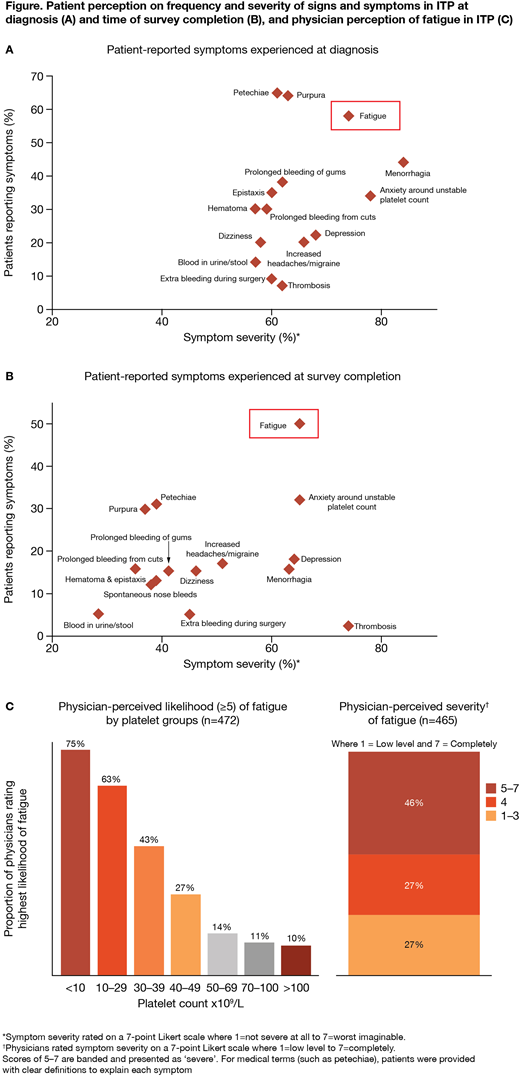
The most frequent pt-reported signs and symptoms at diagnosis and at survey completion (Figure A,B) were petechiae (65%; 31%), bruising (64%; 30%), fatigue (58%; 50%), and anxiety around a stable platelet count (34%; 32%). The most common signs and symptoms MDs reported at diagnosis and overall (ie at any stage) were similar to those reported by pts, with petechiae (82% overall; 83% at diagnosis), purpura (74%; 73%), bleeding of the gums (69%; 70%), and epistaxis (69%; 70%) being the most common; there were few differences between diagnosis and overall. By contrast, fatigue was under-reported by MDs (31% overall; 30% at diagnosis) compared with pt-reported data (58% diagnosis; 50% survey completion).
Pts rated the severity of their current symptoms. Fatigue was one of the most severe pt-reported symptoms, ie scored ≥5 on a 7-point Likert scale (7=worst imaginable severity), both at diagnosis (74%) and survey completion (65%), and remained consistently frequent and severe over time (Figure A,B). Of pts experiencing heavy menstrual bleeding (84%; 63%) and anxiety around a stable platelet count (78%; 65%) these were also reported as severe, although less so at survey completion. Thrombosis, while not common, was considered severe (62%; 74%; Figure A,B). When asked to consider their current symptoms, the three symptoms pts would most like to resolve were heavy menstrual bleeding (75%, n=118/158), thrombosis (74%, n=25/34), and fatigue (73%, n=544/743).
MDs perceived several signs and symptoms as having a high impact on pt QoL (scored ≥5 on a 7-point Likert scale; 7=a great deal), with blood in urine/stool (81%), profuse bleeding during surgery (79%), and menorrhagia (78%) considered the most impactful; 59% believed fatigue has a high impact. Overall, 80% of MDs felt ITP symptoms reduce QoL (scored ≥5 on a 7-point Likert scale; 7=a great deal), and 66% believed that ITP-related fatigue reduces QoL. 46% of MDs believe fatigue is severe (scored ≥5 on a 7-point Likert scale; 7=completely fatigued) and believe fatigue increases as platelet levels decrease (Figure C).
Pts are generally satisfied (79% satisfaction) with their MD's management of their disease, and overall satisfaction between pts and MDs was high regarding communication (80% vs 88%), management (79% vs 86%), and understanding of treatment goals (77% vs 90%).
Summary/conclusions: The pt-reported symptom with highest frequency and greatest severity both at diagnosis and survey completion was fatigue. MDs who participated were experienced with treating ITP and believed fatigue would greatly affect pts, nonetheless MDs did not consider fatigue to be as substantial a problem as pts did. Pts were also concerned about platelet count stability, heavy menstrual bleeding, and thrombosis. Likelihood of fatigue increased as platelet count reduced suggesting fatigue may be intrinsically related to disease activity and could be alleviated by increasing the platelet count. These results indicate that pts and MDs align on overall symptom burden in ITP but highlight that improved understanding and awareness of the relationships between fatigue, platelet count, and QoL is needed.
Kruse:Novartis/ITP: Consultancy; Rigel/ITP: Honoraria; Amgen/ITP: Consultancy. Watson:Novartis: Membership on an entity's Board of Directors or advisory committees. Cooper:Amgen, Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ghanima:Amgen, Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer, BMS, Novartis: Research Funding. Provan:Amgen, Novartis: Honoraria, Research Funding. Arnold:Amgen: Consultancy, Research Funding; UCB: Consultancy; Bristol Myers Squibb: Research Funding; UCB: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Consultancy, Research Funding. Santoro:Bayer, CSL, Novo Nordisk, Pfizer, Shire, Sobi: Other: advisory boards, Speakers Bureau; Grifols, Gilead: Other: advisory boards; Amgen, Glaxo: Speakers Bureau. Tomiyama:Sysmex Corporation: Consultancy; Chugai Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Hakko Kirin Co., Ltd.: Honoraria. Waller:Novartis: Consultancy. Taylor-Stokes:Novartis: Consultancy. Bailey:Novartis: Consultancy. Stankovic:Novartis: Employment. Bussel:Novartis: Consultancy, Research Funding; Uptodate: Honoraria; Rigel: Consultancy, Research Funding; Momenta: Consultancy; Protalex: Consultancy; Amgen Inc.: Consultancy, Research Funding; Prophylix: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.